Atrial fibrillation in a dish enables drug discovery, personalized medicine
The irregular heart rhythm called atrial fibrillation affects more than 30 million people globally, leading to stroke and heart failure in severe cases. But the condition remains difficult to treat, with less than half of patients responding to traditional medications.
Now, a University of Illinois Chicago collaboration bridging the College of Medicine and College of Engineering offers a powerful new laboratory approach for understanding atrial fibrillation and developing novel and personalized treatments.
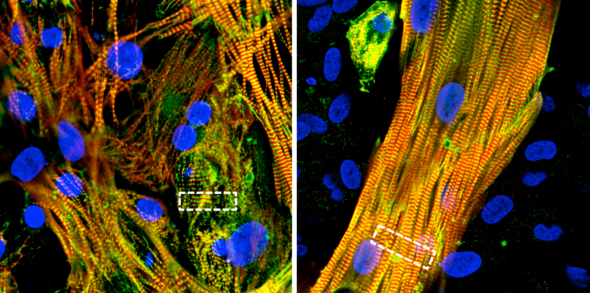
The research, published in Science Advances, describes a new cell patterning process that recreates the structure and activity of major cell types in the heart’s atrial chambers. This laboratory model will allow researchers to not only study atrial fibrillation at a cellular level and conduct high-throughput drug screening but also determine the cause of the condition due to the genetics of individual patients or families.
“This model is going to be incredibly useful for developing more effective personalized treatments for patients and for understanding atrial maturation,” said Dr. Dawood Darbar, professor of medicine and pharmacology and chief of cardiology at UIC.
The model, co-developed by the research groups of Darbar and Salman Khetani, professor and director of graduate studies of biomedical engineering, uses pluripotent stem cells taken from adult patients and grows them into atrial cardiomyocytes — the cells that form heart muscles.
Previous attempts to use these stem cells had only produced immature cells that failed to mimic the behavior of the adult heart. Khetani and Darbar’s groups solved the issue by combining two methods.
The first was “micropatterning” the initial layout of stem cells using lithographic methods, similar to how engineers assemble the microscopic components of computer chips in the semiconductor industry. Researchers also added another heart cell type, called atrial fibroblasts, to the dish during the maturation process.
The results were groundbreaking, the researchers said. The stem cells not only matured into adult atrial cells with normal function, but they also spontaneously formed complex structures that mimicked how muscle cells naturally align to contract in sync in the heart.
“They formed these three-dimensional layers with cells stacked on top of each other, which is how they would be in the heart,” Khetani said. “That’s the beauty of nature: provide the initial conditions, get out of the way, and let nature do its job.”
Several features of the model make it ideal for identifying the causes of atrial fibrillation and testing new treatments. Cells express their typical ion channels and other proteins that mediate heart contraction, so researchers can examine how each contributes to the abnormal rhythm of atrial fibrillation. Cultures grown from stem cells taken from patients with atrial fibrillation exhibited aberrant behaviors that could be studied to determine the genetic cause of the cellular abnormalities.
For scalability, the researchers developed the process to work in multi-well plastic or glass plates used in high-throughput drug screening, where laboratories rapidly test hundreds or thousands of potential treatments at once. The cells also survive for over six weeks, allowing for chronic drug treatment that more closely replicates clinical use for patients with atrial fibrillation.
These features will also help test and apply new medical technologies for atrial fibrillation, including gene editing and personalized therapies. Cells from a patient with the condition could be analyzed in the laboratory for the most effective treatment to guide clinical care, and genetic therapies such as those using CRISPR could fix dangerous mutations before they produce life-threatening symptoms.
“Imagine you have a screen of interfering RNAs to fix atrial fibrillation in specific patient populations,” Darbar said. “I think there’s a lot of potential here, and we’re just scratching the surface as to what this model can be used for.”
The Science Advances paper was first-authored by PhD student Grace Brown and postdoctoral researcher Yong-Duk Han of the UIC Department of Biomedical Engineering and members of the Khetani laboratory. Additional co-authors include Ashlin Michell, Olivia Ly and Emanuele Spanghero from UIC and Drs. Carlos Vanoye and Alfred L. George Jr. from the pharmacology department at Northwestern University Feinberg School of Medicine.
Categories
Health Sciences Colleges, Research, UIC today
Topics
atrial fibrillation, cardiology, engineering, personalized medicine
