Study offers new perspective on chemical interactions that govern metals’ structure
What determines the structures of simple metals has been a subject of a longstanding debate among researchers, and findings from a new study appearing in the Proceedings of the National Academy of Sciences add new information to consider in the discussion.

By analyzing the electronic properties of metals in various lattices over a broad range of sizes and geometries, University of Illinois Chicago professor Russell Hemley and collaborators shifted from a “physics” band-structure point of view to a “chemical” perspective to find highly complex structures that emerge at high pressures in these materials.
They proposed a new theory that can explain why a particular structure forms for almost all metals in the periodic table.
“This theory is based on our finding that the electrons in many metals occupy what we call quasi-atom orbitals — the local quantum orbitals centered at the voids between the atoms. In large measure, it is the chemical interactions between localized electrons that control the structure of the metals,” said Hemley, College of Liberal Arts and Sciences Distinguished Chair in the Natural Sciences.
The result contrasts with the traditional view that structures and other properties are determined by the extended electronic states, which behave close to what is known as a free electron gas. However, the structure preference of classes of metals across the periodic table cannot be explained by this “physics,” or band structure point of view.
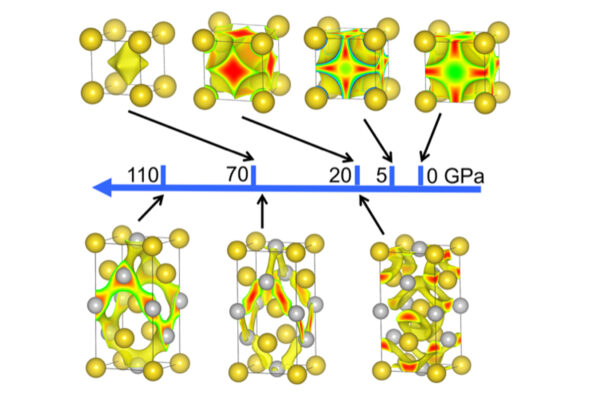
“Our work reveals that the localized electrons and their chemical interactions are key factors in determining the crystal structures of many metals,” Hemley said.
The findings have implications for the behavior of chemically complex materials, including alloys, intermetallics, hydrides, ionic compounds and two-dimensional materials.
One reason the research team is particularly excited about this work is that the idea originated from the need for a simple “chemical” explanation for the existence of high-pressure electrides, a surprising phenomenon that includes, for example, the fact that some alkali metals become transparent insulators under pressure.
“This is an example of how the study of matter under extreme conditions can inform us about chemistry and materials under normal or more familiar conditions. Studies of materials at high pressure can reveal chemical features that might be otherwise neglected,” said Hemley, who holds appointments in UIC’s departments of physics, chemistry, and earth and environmental sciences.
The research was supported by National Science Foundation grants (DMR-1848141, OAC-2117956, DMR-2104881), the American Chemical Society Petroleum Research Fund (50249-UNI6), a California State University Research, Scholarship and Creative Activity award, and the Department of Energy (DE-SC0020340, DE-NA0003975). Computational resources were provided by the Extreme Science and Engineering Discovery Environment (Teragrid-DMR130005).
Co-first authors of the report are Yuanhui Sun of California State University Northridge, Lei Zhao of Southwest Petroleum University and University of Electronic Science and Technology of China, and Chris Pickard of the University of Cambridge. Other authors include Yonghao Zheng of the University of Electronic Science and Technology of China, and Maosheng of Miao, California State University Northridge and the University of California Santa Barbara.
